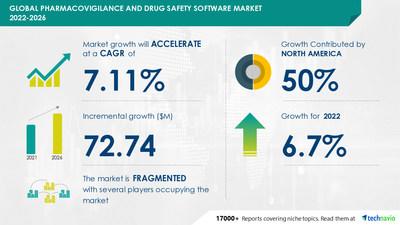
NEW YORK, May 11, 2022 /PRNewswire/ — The global pharmacovigilance and drug safety software market size is expected to grow by $72.74 million from 2021 to 2026, at a CAGR of 7.11% as per the latest market report by Technavio. 50% of the market’s growth will originate from North America during the forecast period. The US is the key market for pharmacovigilance and drug safety software in North America. Market growth in this region will be faster than the growth of the market in other regions. The increase in the number of product recalls will facilitate the pharmacovigilance and drug safety software market growth in North America over the forecast period

For more insights on the market share of various regions – Download a sample report in MINUTES
Read the 120-page report with TOC on “Pharmacovigilance and Drug Safety Software Market Analysis Report by End-user (Pharmaceutical and biotechnology companies, Contract research organization, and Business process outsourcing) and Geography (North America, Europe, Asiaand Rest of World (ROW)), and the Segment Forecasts,2022-2026″. Gain competitive intelligence about market leaders. Track key industry opportunities, trends and threats. Information on marketing, brand, strategy and market development, sales, and supply functions. https://www.technavio.com/report/report/pharmacovigilance-and-drug-safety-software-market-industry-analysis
Pharmacovigilance and Drug Safety Software Market: Vendor Analysis
The pharmacovigilance and drug safety software market is fragmented and the vendors are deploying growth strategies such as focusing on software innovations and are increasing existing software capabilities to expand their presence and share in the market to compete in the market. The pharmacovigilance and drug safety software market report offer information on several market vendors, including AB Cube SARL, Accenture Plc, Advera Health Analytics Inc., ArisGlobal LLC, BaseCon AS, Clarivate PLC, Cognizant Technology Solutions Corp., Ennov, EXTEDO GmbH, Honeywell International Inc., Indegene Pvt. Ltd., IQVIA Holdings Inc., Max Application Srl, Oracle Corp., Pegasystems Inc., Sarjen Systems Pvt. Ltd., United BioSource LLC, Veeva Systems Inc., and Wipro Ltd. among others.
To know about all major vendor offerings – Download a sample now!
Pharmacovigilance and Drug Safety Software Market: Drivers & Challenges
The key factor driving growth in the pharmacovigilance and drug safety software market is the rising incidence rates of adverse drug events. There has been a global surge in the incidence of unwanted and undesirable effects of drugs during their clinical use. This can be attributed to the rising consumption of drugs worldwide and the unwanted medical occurrence and side effects during the treatment, which can result in the discontinuation or modification of drugs. This is a major concern for pharmaceutical manufacturers. Fast adoption of pharmacovigilance and drug safety software products among pharmaceutical and life science manufacturers has been observed owing to the increasing incidence of adverse drug events. The rising geriatric population is expected to further increase the number of patients encountering adverse drug events, which will create a rise in demand for pharmacovigilance and drug safety software. This increase in the geriatric population is anticipated to arise the incidence of adverse drug events, which will positively impact the demand for drug safety software.
However, the high cost of ownership, installation, and maintenance will be a major challenge for the pharmacovigilance and drug safety software market during the forecast period. The cost involved in the on-premises and cloud-based software solutions can be categorized into three major categories: initial capital cost (initial cost), deployment cost, and maintenance cost (ongoing and ongoing personnel cost). All these categories are combined to form the total deployment cost. The low adoption of pharmacovigilance and data safety software among small- and medium-scale business enterprises can be observed across the market due to the high ownership, installation, and maintenance costs. The cost of ownership of on-premises software is even higher than cloud-based solutions. On-premises software for pharmacovigilance and drug safety also involves the cost of hardware, network, facility, and security, which makes them highly-priced in the market. Small-scale pharmaceutical manufacturers who do not rely on large volumes of drug safety solutions restrict them towards the purchase of personnel drug safety software, which hampers the market growth.
To know about other drivers & challenges – Download a sample now!
Pharmacovigilance And Drug Safety Software Market: Segmentation Analysis
End-user Outlook (Revenue, USD mn, 2021-2026)
-
Pharmaceutical and biotechnology companies – size and forecast 2021-2026
-
Contract research organization – size and forecast 2021-2026
-
Business process outsourcing – size and forecast 2021-2026
Geography Outlook (Revenue, USD mn, 2021-2026)
-
North America – size and forecast 2021-2026
-
Europe – size and forecast 2021-2026
-
Asia – size and forecast 2021-2026
-
Rest of World (ROW) – size and forecast 2021-2026
To know about the contribution of each segment – Grab an Exclusive Sample Report
Don’t wait, Make a strategic approach & boost your business goals with our Pharmacovigilance and Drug Safety Software Market ForecastReport- Buy Now!
Related Reports:
-
The predicted growth of the healthcare information software market share from 2021 to 2026 is USD 11.76 billion at a progressing CAGR of 7.9%. Download a sample now!
-
the cancer registry software market share is expected to increase by USD 54.20 million from 2020 to 2025, and the market’s growth momentum will accelerate at a CAGR of 11.19%. Download a sample now!
|
Pharmacovigilance and Drug Safety Software Market Scope |
|
|
Report Coverage |
Details |
|
page number |
120 |
|
base year |
2021 |
|
Forecast period |
2022-2026 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 7.11% |
|
Market growth 2022-2026 |
$72.74 million |
|
Market structure |
Fragmented |
|
YoY growth (%) |
6.7 |
|
performing market contribution |
North America at 50% |
|
competitive landscape |
Leading companies, competitive strategies, consumer engagement scope |
|
Companies profiled |
AB Cube SARL, Accenture Plc, Advera Health Analytics Inc., ArisGlobal LLC, BaseCon AS, Clarivate PLC, Cognizant Technology Solutions Corp., Ennov, EXTEDO GmbH, Honeywell International Inc., Indegene Pvt. Ltd., IQVIA Holdings Inc., Max Application Srl, Oracle Corp., Pegasystems Inc., Sarjen Systems Pvt. Ltd., United BioSource LLC, Veeva Systems Inc., and Wipro Ltd. |
|
Market Dynamics |
Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID 19 impact and future consumer dynamics, market condition analysis for the forecast period, |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get customized segments. |
Table of Contents
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Five Forces Analysis
5 Market Segmentation by End-user
6 Customer Landscape
7Geographic Landscape
8 Drivers, Challenges, and Trends
9 Vendor Landscape
10 VendorAnalysis
11 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
contacts
Technavio Research
Jesse Mayda
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
E-mail: [email protected]
website: www.technavio.com/


View original content to download multimedia:https://www.prnewswire.com/news-releases/pharmacovigilance-and-drug-safety-software-market—50-of-growth-to-originate-from-north-america –evolving-opportunities-with-ab-cube-sarl–oracle-corp-technavio-301544085.html
SOURCE Technavio